Your Read is on the Way
Every Story Matters
Every Story Matters
The Hydropower Boom in Africa: A Green Energy Revolution Africa is tapping into its immense hydropower potential, ushering in an era of renewable energy. With monumental projects like Ethiopia’s Grand Ethiopian Renaissance Dam (GERD) and the Inga Dams in the Democratic Republic of Congo, the continent is gearing up to address its energy demands sustainably while driving economic growth.
Northern Kenya is a region rich in resources, cultural diversity, and strategic trade potential, yet it remains underutilized in the national development agenda.

Can AI Help cure HIV AIDS in 2025

Why Ruiru is Almost Dominating Thika in 2025

Mathare Exposed! Discover Mathare-Nairobi through an immersive ground and aerial Tour- HD

Bullet Bras Evolution || Where did Bullet Bras go to?
Breaking the Chains of Insatiable Hunger: FDA Approves First Therapy for Prader-Willi Syndrome
In a groundbreaking development, the U.S. Food and Drug Administration (FDA) has officially approved Vykat XR, the first-ever treatment designed to address hyperphagia—an uncontrollable and persistent hunger—in individuals with Prader-Willi syndrome (PWS). This milestone represents a significant leap forward in the treatment of this rare genetic disorder, offering new hope to patients and their families.
Prader-Willi syndrome affects approximately 50,000 people in the United States alone, with tens of thousands more worldwide. For decades, there has been no FDA-approved medication specifically targeting the core symptoms of PWS, leaving patients and caregivers struggling to manage the physical and emotional toll of the condition. With the approval of Vykat XR, the medical community now has a powerful tool to help mitigate one of the most challenging aspects of PWS, providing patients with a chance at a better quality of life.
A Lifeline for Those Battling Unending Hunger
Prader-Willi syndrome is a complex and life-altering genetic disorder that affects multiple systems in the body. One of the most distressing and defining symptoms of PWS is hyperphagia, a chronic feeling of hunger that is never satisfied, regardless of how much the person eats. This persistent drive to eat often leads to severe obesity, which can trigger a host of related health complications, including diabetes, cardiovascular disease, and respiratory problems. Beyond the physical manifestations, individuals with PWS frequently experience behavioral issues, including anxiety, temper outbursts, and obsessive-compulsive tendencies. Cognitive impairments and developmental delays are also common, requiring lifelong support and care.
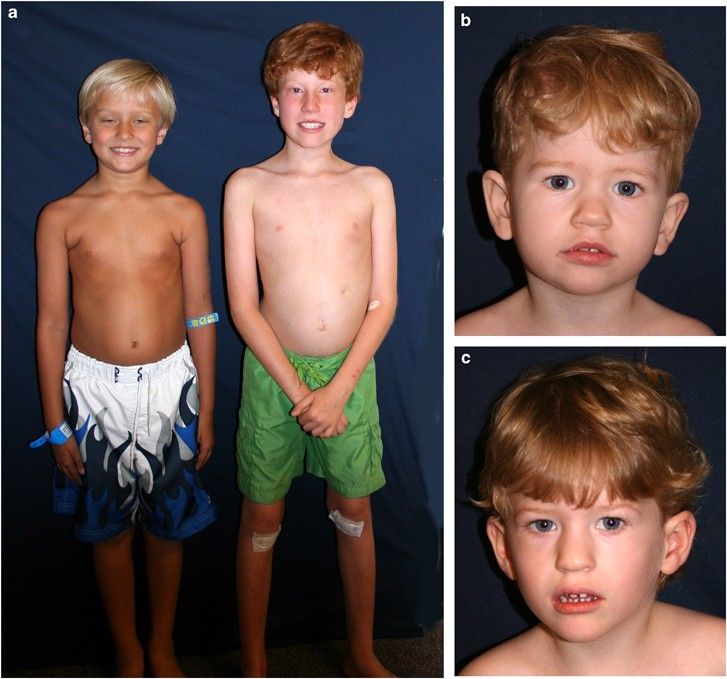
The unrelenting hunger associated with PWS not only affects physical health but also impacts emotional well-being and social interactions, creating a significant burden on both patients and their families. Without effective treatment, the average life expectancy for individuals with PWS ranges from 21 to 29 years, making the approval of Vykat XR a potentially life-extending breakthrough. By specifically targeting the neurological mechanisms behind hyperphagia, this new therapy offers a transformative solution to one of the most distressing aspects of the condition, providing relief for both patients and caregivers.
The Journey to Approval
The road to FDA approval for Vykat XR was marked by years of rigorous research and clinical trials conducted by Soleno Therapeutics, the pharmaceutical company behind the treatment. Early-phase trials produced mixed results, raising questions about the drug's effectiveness. However, a pivotal long-term extension study provided compelling evidence of the drug's efficacy. Patients who received the medication for at least one year demonstrated significant and sustained reductions in hyperphagia, with many participants experiencing improvements in overall behavior and quality of life.
These findings were critical in securing FDA approval, as they highlighted the medication's potential to address a long-standing unmet medical need. The approval process also included extensive safety evaluations, ensuring that Vykat XR met the FDA’s strict standards for both efficacy and patient well-being. The success of Vykat XR represents more than just a scientific achievement—it reflects the persistence of the PWS community, which has long advocated for better treatments and increased research funding. With the green light from the FDA, patients now have access to a targeted therapy that could dramatically alter the course of their lives.
Looking Ahead
Vykat XR is expected to become available in the United States starting in April 2025, marking the beginning of a new era in the treatment of Prader-Willi syndrome. The medication will be approved for use in patients aged four years and older, making it accessible to a broad range of those living with PWS. However, the therapy comes at a steep price—$466,200 per year—which may present a barrier to widespread adoption. In response, patient advocacy groups and healthcare organizations are actively working to expand access and secure insurance coverage, ensuring that families in need can afford the life-changing treatment.
Beyond the U.S., efforts are also underway to obtain international regulatory approvals, potentially bringing the therapy to patients around the world. While the cost and logistical challenges remain substantial, the approval of Vykat XR signifies a monumental step forward in the fight against Prader-Willi syndrome. For the first time, patients and their families can envision a future where the relentless hunger and the associated health risks can be effectively managed, offering new hope for longer, healthier, and more fulfilling lives.
0 comments